Abstract
Introduction: Children and young adults with B-lymphoblastic leukemia (B-ALL) who relapse with CD19 antigen loss after CD19-targeted CAR T-cell therapy have a dismal prognosis, with a median overall survival of 9.7 months. Novel immunotherapies are urgently needed for this population. CD22 can serve as a potential alternative target. In our initial pilot trial of CD22-targeted/4-1BB CAR T cells, clinical activity was unexpectedly poor. Based on additional preclinical work demonstrating that shorter scFv linkers improve T-cell effector function, a novel CD22/4-1BB CAR construct with a short scFv linker (CART22-65s) was developed and investigated in a phase 1 study (NCT02650414).
Methods: After fludarabine/cyclophosphamide lymphodepletion, patients (pts) were infused with CART22-65s using a 3-day fractionated dosing scheme with the 2nd/3rd doses held for early signs of cytokine release syndrome (CRS). The primary endpoint was safety. Key secondary endpoints were feasibility, efficacy, and CAR T cell expansion and persistence.
Results: Nineteen pts were enrolled and had a product successfully manufactured. Of 17 pts infused (median age, 16 years; range 3-28 years), all had CD19-negative, relapsed disease after prior CD19-directed therapy (CART19, n=16; blinatumomab, n=1); 8 had prior inotuzumab; 9 had prior stem cell transplant (SCT); and 3 had multiple prior SCTs. Median bone marrow blasts immediately pre-infusion was 31% (range, 0-99%); 8 patients had >75% blasts. The median cell dose infused was 4x106 CART22-65s cells/kg (range, 0.8 to 10 x 106).
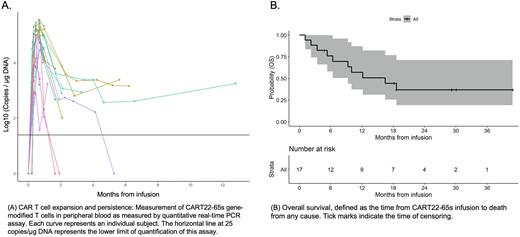
In vivo cellular kinetics by qPCR demonstrated robust initial CAR T cell proliferation peaking at a median of 20 days post-infusion, and persistence that correlated with clinical response (Figure A). At day 28, 13/17 (77%) pts achieved a complete remission (CR) of which 10 (77%) had undetectable minimal residual disease (uMRD) by flow cytometry. One additional pt cleared MRD at month 2 without intervention. Notably, 4/5 (80%) pts refractory to prior inotuzumab achieved a CR with CART22-65s. Of 11 pts with a best response of uMRD CR, 5 proceeded to consolidative SCT, including 3 with prior SCT: 2 remain in CR post-SCT, 2 are in CR after a brief course of alternative therapy for emergence of MRD, and 1 had a CD22+ relapse post-SCT. Of 6 pts with uMRD CR who did not proceed to SCT (5 had a prior SCT), 5 experienced relapsed disease with a range of CD22 expression and 1 remains in CR 30 months after infusion with ongoing CAR T persistence. Of 2 pts with MRD+ CR (1 CD22+, 1 CD22-), both progressed to morphologic relapse; one received alternative therapy prior to progression. Of 4 pts with non-response, all died of disease within 9 months. Thus, with a median follow-up of 29 months, median relapse-free, event-free, and overall survival were 5.3 (95% CI, 1.9-NR), 5.8 (95% CI, 2.1-NR), and 16.5 (95% CI, 9.3-NR) months, respectively (Figure B). In addition, 2 patients received CART22-65s retreatment for CD22+ relapsed disease after initial infusion. Both engrafted, but only 1 achieved uMRD CR.
CRS was observed in 15 pts (grade 1, n=10; grade 2, n=5), and mild neurotoxicity in 6 pts (grade 1, n=5; grade 2, n=1). In the retreatment setting, 1 pt developed grade 3 CRS, which resolved, and grade 3 encephalopathy/hypotonia, but died of progressive disease prior to resolution. Autopsy findings were consistent with leukomyelopathy of the spinal cord, which was inconclusive in its relation to CART22-65s. Other SAEs included: (1) platelet refractoriness and recurrent, severe inflammatory reactions to platelet transfusions in 1 pt that improved with corticosteroids and anakinra; and (2) delayed hemophagocytic lymphohistiocytosis in 1 pt after CART22-65s retreatment that resolved without intervention.
Conclusion: CART22-65s induced remissions in 77% of children and young adults with highly refractory, CD19-negative B-ALL, including in 4/5 pts refractory to prior CD22-directed therapy. Manufacturing was successful, and the overall toxicity profile was favorable even in pts with high disease burden. Several uncommon toxicities were observed, but the only grade 3 CRS and neurotoxicity events occurred in the retreatment setting. Our observation of high initial response rates, but later recurrences, highlights the need to use CART22-65s as part of combination therapy. These promising preliminary data provide support for a combined CD19/22 approach using the CART22-65s construct.
Disclosures
Brogdon:Novartis: Current Employment, Current holder of stock options in a privately-held company, Patents & Royalties. Engels:Novartis: Ended employment in the past 24 months, Other: Holds stocks and patents; Miltenyi Biotec: Current Employment. Rheingold:Pfizer: Consultancy. Frey:Sana Biotechnology, Kite Pharma, and Syndax Pharmaceuticals: Consultancy; Novartis: Research Funding. Maude:Wugen: Research Funding; Novartis Pharmaceuticals: Consultancy, Other: study steering committee, Patents & Royalties: Patent pending and licensed to Novartis Pharmaceuticals for PCT/US2017/044425: Combination Therapies of Car and PD-1 Inhibitors , Research Funding. Grupp:Carisma Therapeutics: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kite: Research Funding; Vertex: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Jazz: Membership on an entity's Board of Directors or advisory committees, Research Funding; Servier: Research Funding; Roche: Consultancy; GSK: Consultancy; CBMG: Consultancy; Eureka: Consultancy; Amerisource: Consultancy; Janssen: Consultancy; Adaptimmune: Membership on an entity's Board of Directors or advisory committees; Cellectis: Membership on an entity's Board of Directors or advisory committees; Juno: Membership on an entity's Board of Directors or advisory committees; Vertex: Membership on an entity's Board of Directors or advisory committees; Alogene: Membership on an entity's Board of Directors or advisory committees; Cabaletta: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal